Project & Team
Clinical Project Description of CMT-MOD Biomarker Study
Recruitment
Patient Recruitment only via E-Mail:
contact@cmt-mod.org
Sponsor
This study is sponsored by the AFMTÉLÉTON.
Study Title
Full Title: A multi-omic approach to the identification of novel biomarkers in early Charcot-Marie-Tooth 1A disease (CMT1A) (CMT-MODs)
Clinical Phase
This is a biomarker study, not associated with a specific clinical phase.
Coordinators
Lead Coordinator:
Univ.-Prof. Dr. med. Michael W. Sereda
University Medical Centre Göttingen (UMG), Department of Neurology
Address: Robert-Koch-Str. 40, 37075 Göttingen, Germany
Email: mwsereda@med.uni-goettingen.de or sereda@mpinat.mpg.de
Co-Coordinator:
Prof. Shahram Attarian, M.D., Ph.D.
Neuromuscular Disease and ALS Reference Center, Timone University Hospital, Aix-Marseille University
Address: CHU Timone, 264 rue Saint Pierre, 13385 Marseille Cedex 05, France
Email: Shahram.ATTARIAN@ap-hm.fr
Participating Centers
The project involves multiple research centers led by principal investigators, including:
University Medical Centre Göttingen (UMG), Germany
Principal Investigator: Univ.-Prof. Dr. Michael Sereda
Timone University Hospital, Aix-Marseille University, France
Principal Investigator: Prof. Shahram Attarian

Research Partners and Collaborators
Research Partners (Direct Funding)
These Institutions are directly involved in the project´s core activities and receive funding to support their work.
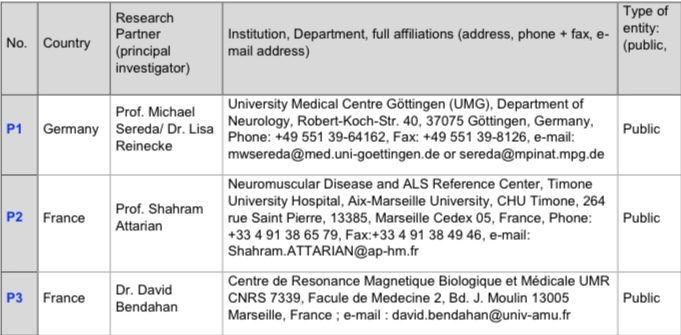
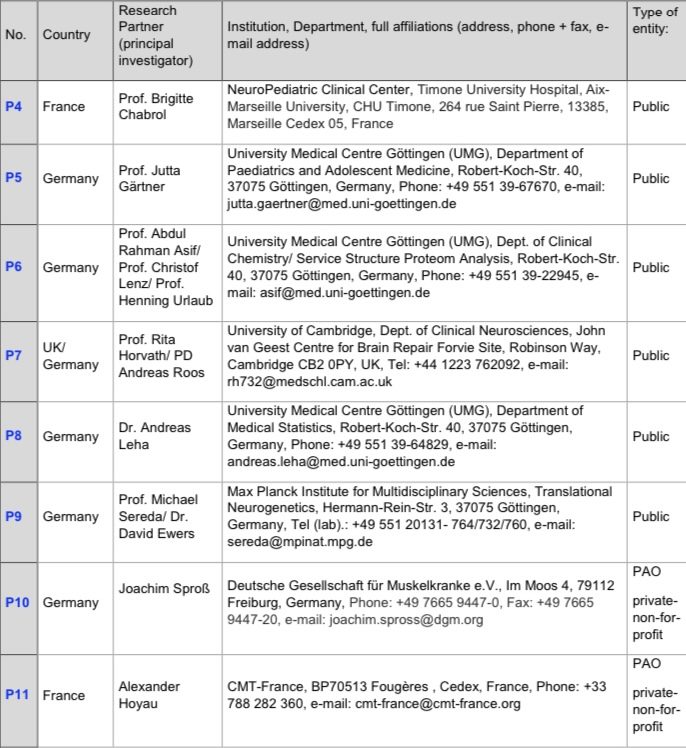
Collaborating Partners (Indirect funding via P1 or P2)
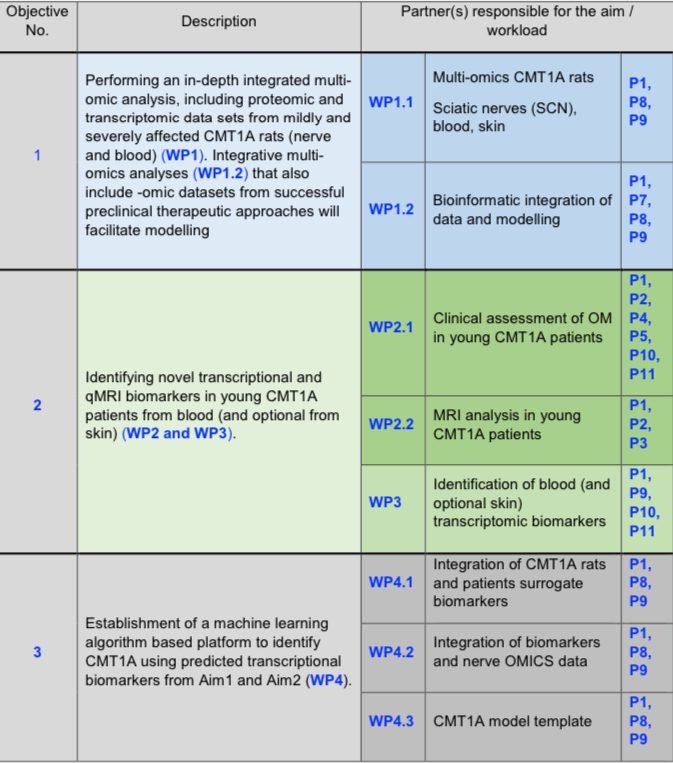
Objectives (primary and secondary)
Estimated Study Duration
Inclusion Period: 6 months
Study Duration: 12 months
Total Timeframe: 18 months (from first patient enrolled to last patient completed).
©Copyright. All rights reserved.
Wir benötigen Ihre Zustimmung zum Laden der Übersetzungen
Wir nutzen einen Drittanbieter-Service, um den Inhalt der Website zu übersetzen, der möglicherweise Daten über Ihre Aktivitäten sammelt. Bitte überprüfen Sie die Details in der Datenschutzerklärung und akzeptieren Sie den Dienst, um die Übersetzungen zu sehen.
